-
Scientific & Basic Research
- Primary Media
- Classical & Basal Cell Culture Media
- Drosophila Cell Culture Media
- DMEM Cell Culture Media
- RPMI 1640 Cell Culture Media
- DMEM/F-12 Cell Culture Media
- F-12 Cell Culture Media
- MEM Cell Culture Media
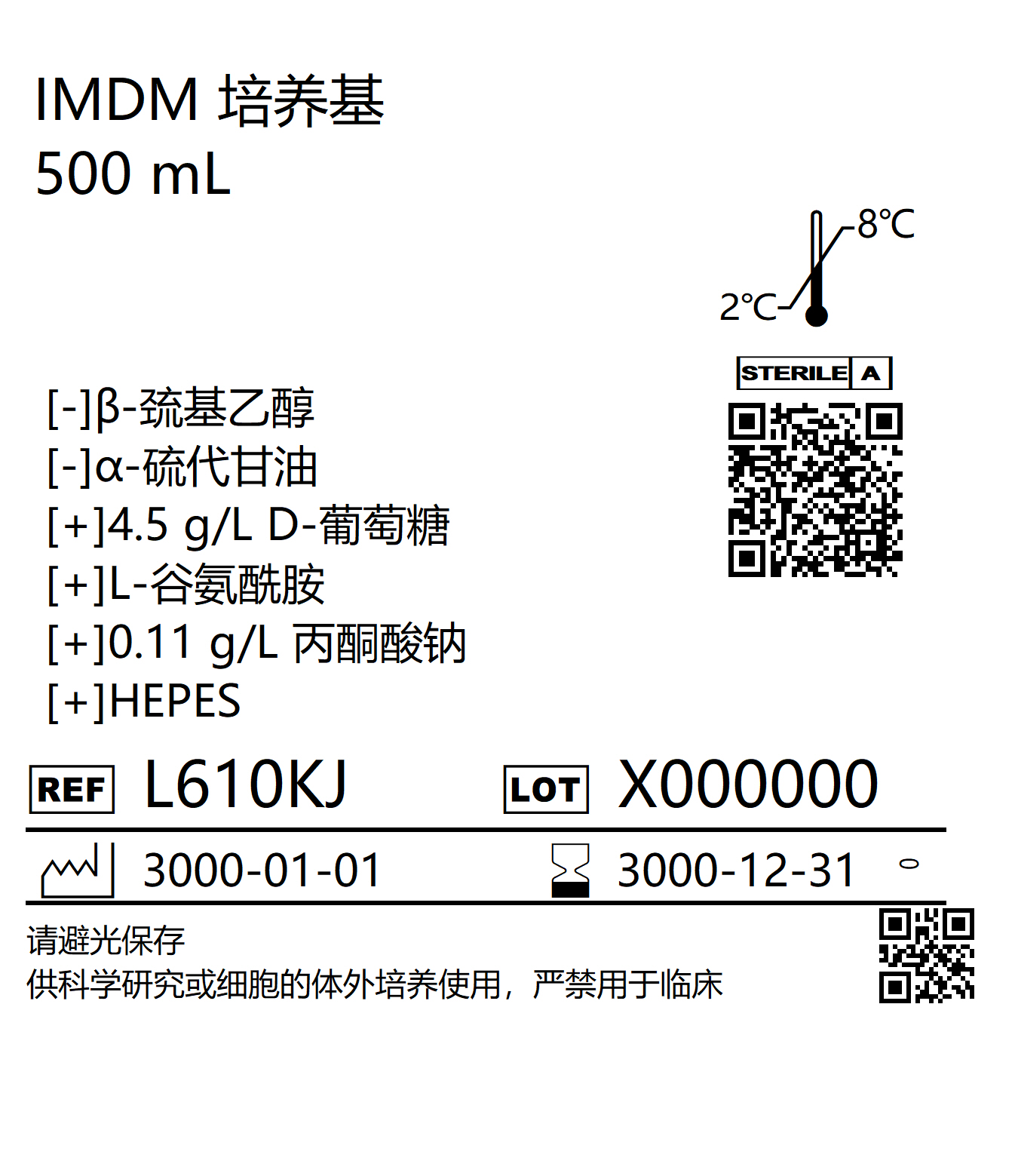
- IMDM Cell Culture Media
- M199 Cell Culture Media
- L-15 Cell Culture Media
- McCoy's 5A Cell Culture Media
- CO2 Independent Media
- Williams Cell Culture Media
- Waymouth MB 752/1 Cell Culture Media
- MCDB 131 Cell Culture Media
- MCDB 153 Cell Culture Media
- Insect Cell Culture Media
- M2 Media
-
Cell Culture Reagents
- Buffers
- Life Sicence Buffers
- Antibiotics, Antimycotics
- Dissociation Reagents
- Cell Culture Supplements
- ITS
- L-glutamine
- L-alanyl-L-glutamine
- MEM Essential Amino Acids Solution
- MEM Non-Essential Amino Acids Solution
- MEM Vitamin Solution
- Sodium Pyruvate Solution
- HT Supplement
- HAT Supplement
- GS Supplement
- Anti-Clumping Agent
- Chemical Defined Lipid Concentrate
- Recombinant Human Serum Albumin (rHSA) Solution
- Plant Derived Synthetic Cholesterol
- Plant Protein Hydrolysate Solution
- CEPT Supplement
- Y-27632
- Bovine Serum Albumin
- Recombinant Human Insulin
- Recombinant Human Transferrin (HOLO)
- Cryopreservation & Freezing Reagents
- Cell Density Gradient Separation Solutions
- Water for Cell Culture
- Other Cell Culture Reagents
-
Vaccines & Biopharmaceuticals
- Recombinant Protein and Antibiotics Production
- Virus Expression Media
- Vero Cell Serum-Free Media
- MDCK Cell Serum-Free Media
- PK-15 Cell Serum-Free Media
- ST Cell Media
- BHK-21 Cell Media
- MDBK Cell Serum-Free Media
- CEF Cell Media
- Chicken Embryo DF-1 Serum-Free Media
- MSVP Serum-Free Media
- Human Diploid Cell (HDC) Media
- A549 Human Lung Carcinoma Cell Serum-Free Media
- Hela Cell Serum-Free Media
- Mycoplasma Serum-Free Media
-
Cell & Gene Therapy

Online Message 或者发邮件至
info@basalmedia.com
info@basalmedia.com
服务时间: 8:00-17:00
021-37196939
021-37196939